Since the introduction of Project Optimus by the FDA in 2020 [1], it has become more important than ever to ensure that oncology trials consider both efficacy and toxicity from the earliest phase. Similarly, it is key to consider ranges of doses that can be investigated at later stages.
This means that rather than looking for a Maximum Tolerated Dose (MTD) in Phase I and carrying that through for testing at Phase II and onward, Phase I trials should consider efficacy outcomes alongside toxicity outcomes to determine the range of dose levels that could be considered the Optimal Biological Dose and compare these at Phase II against one-another as well as placebo or active comparator to bring through to Phase III trials only the best performing optimal biological dose that could make a real impact for patients against standards of care.
Obviously a standard 3+3 Phase I trial that would take a cohort of patients at ever increasing dose levels until dose limiting toxicities are hit, does not cover such a scheme. Therefore, other methods will need to be considered. Thankfully there are several dose optimizing methods that can cater for the new paradigm brought through by Project Optimus.
One such method is the BOIN12 design, created by Dr R Lin in 2020 [2], this is an adaptation of the BOIN family of methods which combines both Phase I and Phase II trials and utilizes a combined endpoint of efficacy and toxicity.
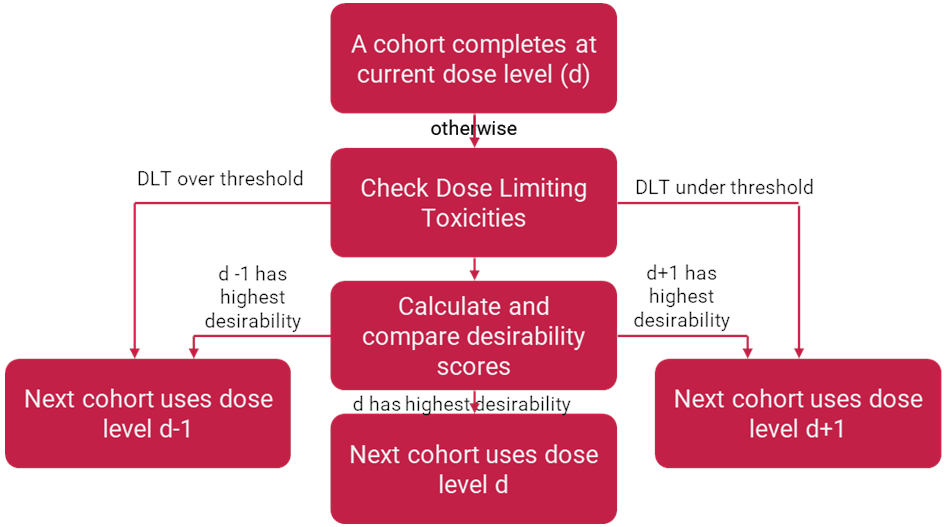
The Phase I element of the trial follows a similar method to the 3+3 design, however as each cohort comes through there is an opportunity for the next cohort to be recruited to either the dose level above, below or the same dose level as had just been recruited to. This decision making is done within the Bayesian framework so all patients at a dose level are considered when deciding what dose level the next cohort will take, that includes the current cohort and any patients from cohorts that have previously been entered at this dose level. This escalation/de-escalation decision is determined by an algorithm based on the acceptable efficacy and toxicity levels for the trial and the outcomes observed at each dose level.
A lower bound of efficacy will be set before the trial starts, this is determined to be the lowest level of efficacy you would want to see from your treatment, this is often set at the current standard of care within the disease of interest, or potentially an acceptable point above what no treatment could do. For example, this could be as low as 10% in rare cancers with no current approved treatments, or 35% if this is the expected rate of response for chemotherapy for this specific cancer in your population of interest.
Similarly, an upper bound of toxicity will be set before the trial starts, this is the highest level of toxicity you would expect to see from patients without raising concerns, this could again be set at standards of care or comparable therapies. For example, this could be 30% of patients receiving a grade 3 or higher adverse effect (AE), if that is the expected rate of severe AEs from chemotherapy for this type of cancer in your given population.
From this set of upper and lower bounds, the BOIN12 algorithm [3] determines the levels of dose-limiting toxicities (DLTs) that would need to be observed within all patients at your current dose level to trigger escalation or de-escalation. If your level of DLTs falls within the boundaries, then the next cohort is decided based on the utility score of the current dose level, the dose level above and the dose level below.
A utility score is a score from 0-100 of the medical utility of a set outcome that can be achieved by a patient. In its simplest form, we take efficacy and toxicity as binary outcomes where a patient can either have an efficacious result from treatment, or not, and can have a toxic event or not. We would score 100 to the best possible outcome (here efficacy without toxicity), and a score of 0 would go to the worst possible outcome (toxicity without efficacy). All other outcome states are given scores determined by which is considered the better outcome as judged by experts. For example, it may be seen that an efficacious result even with toxicity is better than a non-toxic non-efficacious result, and our experts may numerically scale those two states as 60 and 40 respectively
|
Non-toxicity |
Toxicity |
|---|---|---|
Efficacious |
100 |
60 |
Non-Efficacious |
40 |
0 |
Therefore, from this we can rate the overall utility of a dose from the overall outcomes of patients and through comparison to the worst possible acceptable outcome we can determine which potential dose level ranks the most desirable, and that will be the dose level we recruit the next cohort into. The worst possible acceptable outcome is the hypothetical state where we have hit exactly the maximum tolerated level of toxicity and the minimum tolerated level of efficacy, any worse than this state in either direction would make the dose level unacceptable. The BOIN12 algorithm compares all possible outcomes for the number of patients recruited at a specific dose level, comparing and ranking them against this hypothetical worst possible outcome and tabulating such outcomes. Therefore, investigators are able to read off the rank of whatever outcome that dose level, the dose level above and the dose level below have achieved and start the next cohort in the most appropriate level. This means that regardless of the number of patients each level has, they are still comparable. It also means that if a dose level ever achieves a worse ranking than the hypothetical worst, they are given a rank of “E” to indicate that no further patients should be recruited to this dose level.
Once a prespecified number of cohorts have been recruited or a preset number of cohorts have been recruited to a specific dose level (likely indicating a local maximum) then the Phase I part of the trial is concluded and the best performing doses can be taken across for expanded comparison in the Phase II, where patients are randomized to the dose levels of interest. This can be scaled so recruitment is weighted towards the better performing dose levels to maximize the number of patients receiving the currently assumed optimal biological dose. This will be carried out like a standard Phase II trial, but due to the Bayesian nature of the trial and the continuation from the Phase I, any patients recruited to the dose levels that are being carried through in the Phase II can have their results included in the final analysis of the Phase II, supplementing the dataset with extra appropriate information and not wasting results from Phase I.
As can be seen, this methodology clearly follows the Project Optimus ideals by striving constantly to ensure patients in Phase I are always being dosed at what is considered at the time of their dosing to be the optimal dose level and weighting the recruitment in Phase II to put more patients towards this optimal level as well. It also takes into consideration efficacy as a coprimary endpoint throughout and continues to investigate a whole range of dose levels into the Phase II trials to give the best chance for the true biologically optimum dose level to be found.
Utilizing Bayesian methods ensures that no patient’s data is overlooked, and a broader range of understanding and information is used to come to conclusions. Whilst there is more setup involved with tailoring the algorithm to your trial’s needs and determining utility scores, once the initial setup has been achieved, it is a relatively simple algorithm to follow even for non-statisticians since all potential outcomes have been considered within the ranking of desirability scores and can easily be read off a prewritten table.
Whilst sample sizes for a Phase I may be larger when compared to a 3+3 design, the fact that these patients can be carried across to be combined with the Phase II patients can result in fewer patients overall and will hopefully lead to more successful Phase III trials. BOIN12 has also been seen to provide better overdose control and a higher accuracy when identifying optimal biological doses over other model based and model assisted designs.1, 4
However, the fact that the BOIN12 method relies on efficacy outcomes for each cohort before a new cohort can have their dose level selected can mean long waiting times, unless surrogate endpoints (such as indicative biomarker changes) are used. This is a problem encountered by any trial trying to truly follow the ideals of Project Optimus and consider efficacy at an early stage, however this concept does mean that a strong indication of what efficacy is expected to be seen at later phase trials is already known in advance with strong statistical evidence.
Whilst there are always going to be difficulties working under the paradigm of Project Optimus, the BOIN12 method should definitely be considered as a suitable design to achieve its goals.
References
1 FDA Website: https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus
2 Lin et al, BOIN12: Bayesian Optimal Interval Phase I/II Trial Desing for Utility-Based Dose Finding in Immunotherapy and Targeted Therapies. JCO Precis Oncol 4, 1393-1402(2020)
3 MD Anderson BOIN12 algorithm: https://biostatistics.mdanderson.org/shinyapps/BOIN12/



