The early stages of clinical development are critical, and the decisions made here determine not only the efficiency and cost of a program but ultimately whether a promising compound reaches patients at all. As the therapeutic landscape evolves and the demand for innovative trial designs grows, early-phase studies have become an ever-changing and rapidly advancing field.
In this blog, we explore how early-phase clinical trials have evolved over time, from traditional dose-escalation methods to adaptive, model-based approaches, and examine the key trends shaping this dynamic area of drug development.
The Evolution of Early-Phase Design
Over the years, early-phase clinical trials have undergone significant transformation. The traditional “3+3” design, often used for determining the maximum tolerated dose (MTD), is now being joined, and in most cases replaced, by more sophisticated, model-based and adaptive approaches. These methods allow researchers to make better use of accumulating data, identify safe and active doses more efficiently, and reduce the number of patients exposed to suboptimal or overly toxic levels.
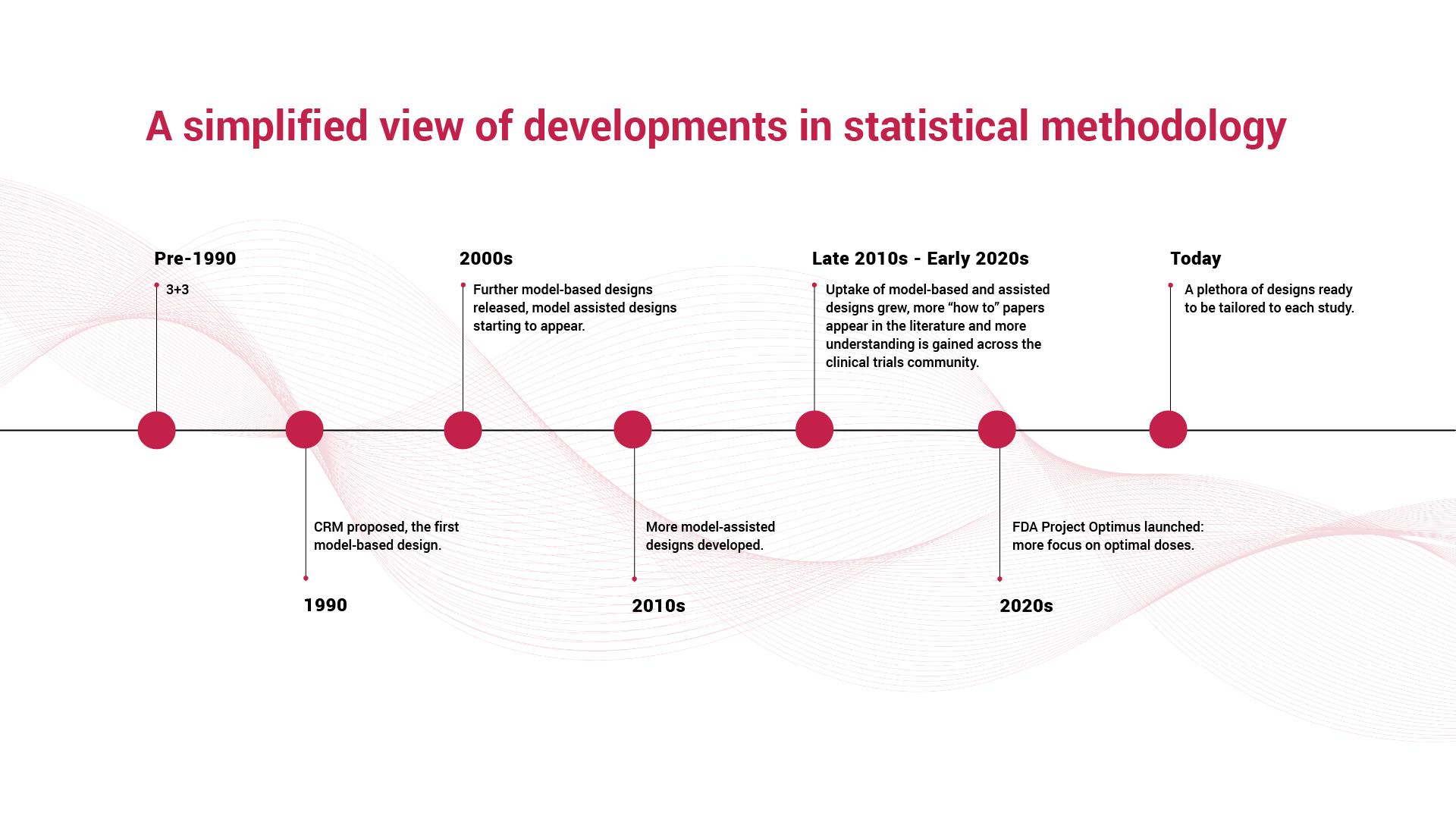
A timeline of methodological progress tells the story clearly: from early rule-based dose-escalation methods to the introduction of the continual reassessment methods (CRM), then further model-based and model-assisted adaptive designs that respond dynamically to observed outcomes. Each advance reflects an effort to improve precision, safety, and decision-making at this crucial stage of development.
Why Change is Necessary
The drive for innovation in early-phase design stems from the complexity of modern therapies. In oncology and other advanced therapeutic areas, understanding the balance between efficacy, tolerability, and biological activity is far more nuanced than in traditional drug development. Relying solely on toxicity to determine the dose(s) for later phase testing often provides an incomplete picture.
As a result, the concept of the Optimal Biological Dose (OBD) has become increasingly relevant. Rather than identifying a single dose at which toxicity is acceptable, sponsors and regulators are focusing on identifying a range of doses that maximize therapeutic benefit while maintaining tolerability. This shift is particularly evident in oncology through initiatives like Project Optimus, which encourages sponsors to explore dose–response relationships more comprehensively before moving to later-phase trials.
In-Progress Study Example: We are currently working with a small biotech on a study that balances flexibility with scientific rigor, enabling informed decisions throughout the trial while maintaining a clear statistical framework. The goal is to identify the optimal dose for further development by considering safety, tolerability, efficacy, PK, and translational data. A dose-expansion approach with backfilling allows additional data collection based on emerging data reviews, with a defined maximum sample size at each dose rather than a fixed enrolment target. This adaptive structure supports efficient progression and robust evidence generation.
Collaboration and Communication: The Foundation of Success
While statistical innovation has been a driving force in this evolution, the human element remains central. Designing and running an early-phase study requires genuine collaboration between clinicians, statisticians, and operational teams. Each brings unique insight, and each question or observation can fundamentally reshape the approach.
That partnership-driven process is essential for navigating the complexity of new designs, interpreting emerging data, and ensuring that decisions made early on are both scientifically and ethically sound.
The Road Ahead
Early-phase trials continue to evolve at pace. As novel therapies emerge and regulatory expectations shift, flexibility, communication, and statistical innovation will remain vital. New designs, new documentation standards (such as SPIRIT [1] and SAPS extensions [2]), and new approaches to data collection, including randomization, back-filling and patient-reported outcomes, (PROs) are all changing what it means to design a robust, informative first-in-human or dose-finding study.
At Phastar, our teams have supported sponsors across oncology and rare disease programs in adopting adaptive, model-based designs that balance safety, efficiency, and regulatory rigor, helping them make better-informed early decisions and accelerate progress to later phases.
References
1. Yap, C., Rekowski, J., Ursino, M., Solovyeva, O., Patel, D., Dimairo, M., Weir, C. J., Chan, A.-W., Jaki, T., Mander, A., Evans, T. J. R., Peck, R., Hayward, K. S., Calvert, M., Rerhou Rantell, K., Lee, S., Kightley, A., Hopewell, S., Ashby, D., Garrett-Mayer, E., Isaacs, J., Golub, R., Kholmanskikh, O., Richards, D. P., Boix, O., Matcham, J., Seymour, L., Ivy, S. P., Marshall, L. V., Hommais, A., Liu, R., Tanaka, Y., Berlin, J., Espinasse, A., & de Bono, J. (2023). Enhancing quality and impact of early phase dose-finding clinical trial protocols: SPIRIT Dose-finding Extension (SPIRIT-DEFINE) guidance. BMJ, 383, e076386. https://doi.org/10.1136/bmj-2023-076386
2. Homer, V., Yap, C., Bond, S., Holmes, J., Stocken, D., Walker, K., Robinson, E. J., Wheeler, G., Brown, S., Hinsley, S., Schipper, M., Weir, C. J., Rantell, K., Prior, T., Yu, L.-M., Kirkpatrick, J., Bedding, A., Gamble, C., & Gaunt, P. (2022). Early phase clinical trials extension to guidelines for the content of statistical analysis plans. BMJ, 376, e068177. https://doi.org/10.1136/bmj-2021-068177



