Introduction
With the launch of Project Optimus, the FDA aims to reform the dose optimization and dose selection paradigm in oncology drug development. The initiative addresses the issue of poorly characterized doses and schedules, which can lead to increased toxicity without additional efficacy.
This transformation necessitates significant changes in how early phase trials are designed and conducted. Phastar presented a webinar on the key messages and strategies drug developers need to know to be ready and prepared to navigate this uncertain new regulatory landscape.
Phastar’s in-house expert statisticians, Giles Partington, MSc, and Sam Hinsley, MSc, joined the webinar to discuss their valuable perspectives on the new guidance and provide strategies to overcome prospective hurdles related to it.
Understanding these considerations and engaging with expert statistician partners early in the process will contribute to the smooth running of your clinical trials, accurate reporting, and achieving the best possible outcomes for your studies and patients.
Project Optimus: Advantages, Disadvantages, and Implications for Clinical Trials
Giles began by providing background to Project Optimus [1], describing the FDA’s emphasis on moving away from maximum tolerated doses (MTDs) in cancer therapies and a move toward a focus on efficacy from phase I.
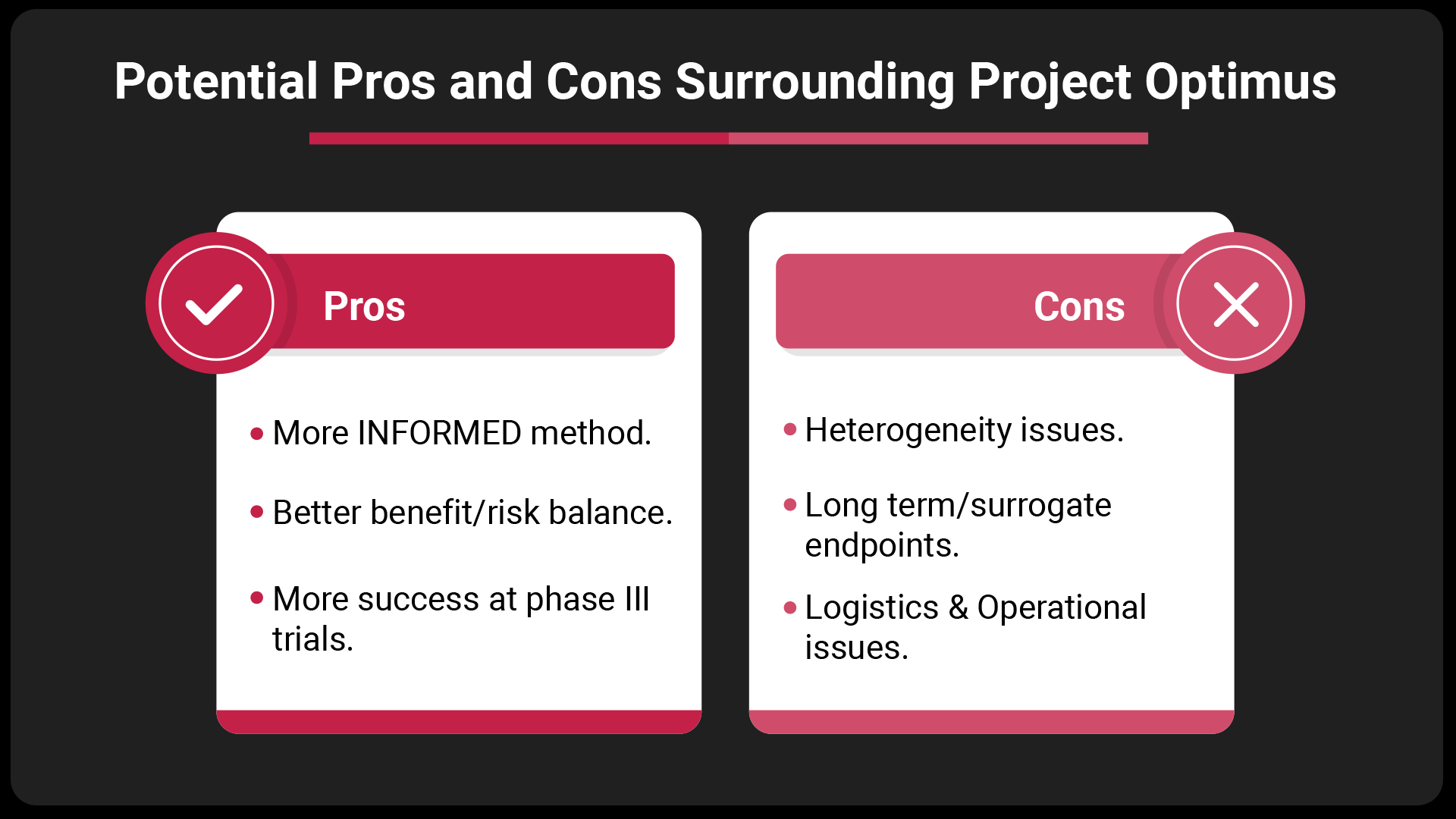
Whilst Project Optimus has many advantages, including a better risk/benefit ratio and increasing success rates at later phases, with any new paradigm there are potential hurdles to overcome.
These include heterogeneity in trials, where both differences within patients, and the disease and treatment pathways can lead to a high variability. Often in phase I, patient numbers are low, and timelines are short, which brings about the possibility of higher failure rates at phase I and II. With the likely need for shorter term surrogate safety/efficacy endpoints, logistic and operational issues must be considered.
It is clear drug developers need to be aware of these challenges and employ expert strategies to overcome them to ensure clinical trial success.
Complying with Project Optimus: the Role of Model Assisted/Based Clinical Trial Designs
Giles discussed how traditional 3+3 designs in clinical trials [2] will no longer provide enough information once Project Optimus is implemented and went on to consider what new methods could be considered instead, that will fulfill the guideline’s criteria.
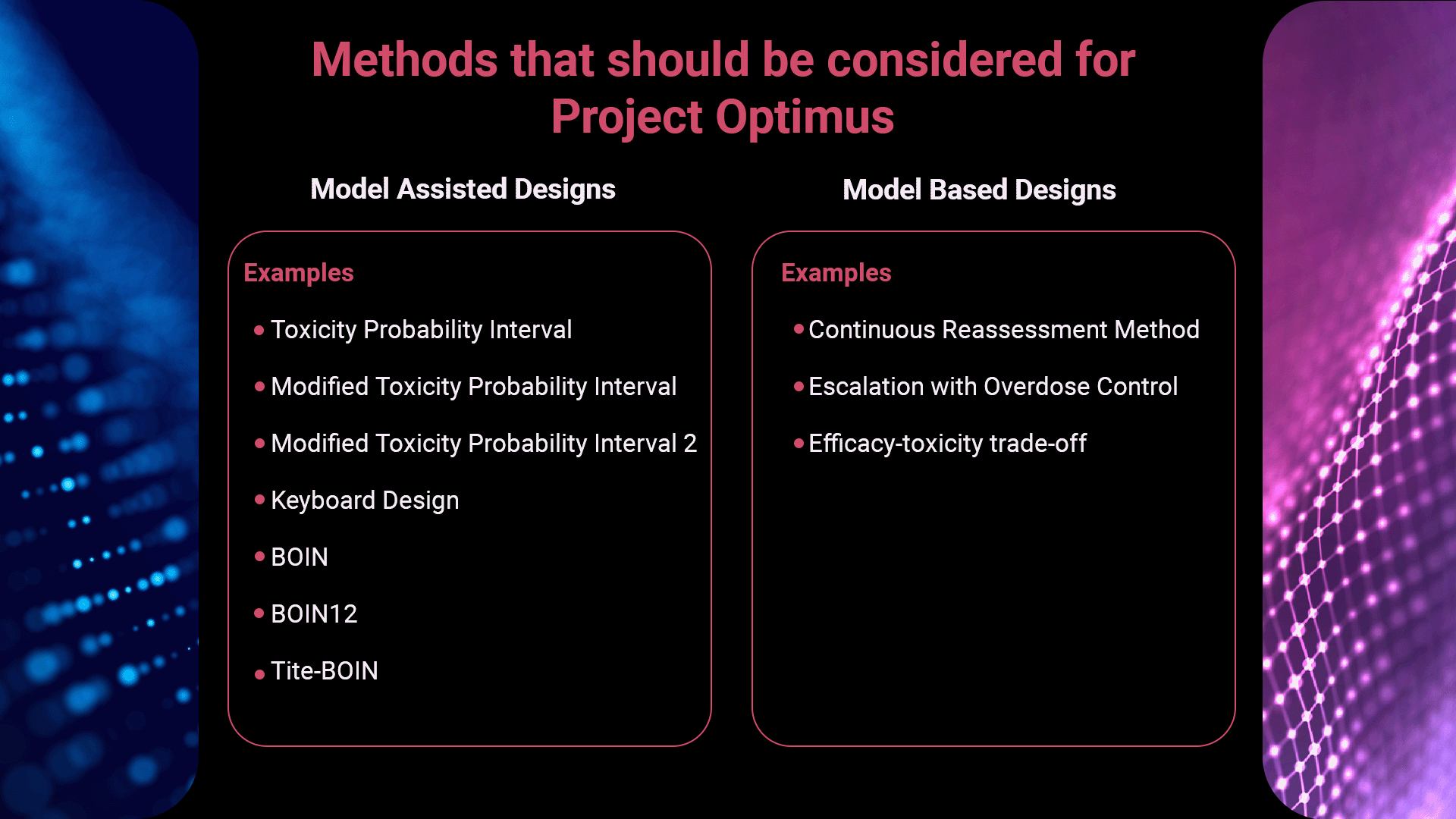
Drug developers should start considering model assisted and model based design options to ensure more rigorous testing in these early phase trials. Model assisted approaches use statistical models to determine pre-defined rules for escalation/de-escalation of dose levels. Model based designs typically model the entire dose-toxicity curve and use this to guide subsequent dose recommendations.
Watch the full webinar for Giles’ detailed walk-through of a BOIN 1-2: Bayesian Optimal Interval Phase I/II Trial Design. [3]
Conclusion: Driving FDA Approvals Through Supported and Strategic Clinical Design
Strategic clinical trial design is a cornerstone of success in drug development, underpinning informed decision-making and advancing patient-centric outcomes.
Collaboration among sponsors, clinical scientists, clinicians, and statisticians is essential from the outset to ensure that trials are appropriate, well-controlled, and have the highest possible probability of success and approval.
About Phastar
As a leading global biometrics contract research organization, we are committed to partnering with stakeholders to navigate clinical design complexities and ensure compliance with Project Optimus. Engaging with statistical experts early on and throughout the process is essential to ensure the best possible outcomes for your clinical trials.
Interested in learning more?
Get in touch with our experts today
Discover more about Bayesian Statistical Approaches in Clinical Trials
References
1. Project Optimus Reforming the dose optimization and dose selection paradigm in oncology, https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus
2. Li, A., & Bergan, R. C. (2020). Clinical trial design: Past, present, and future in the context of big data and precision medicine. Cancer, 15 September. https://doi.org/10.1002/cncr.33205
3. Lin, R., Zhou, Y., Yan, F., Li, D., & Yuan, Y. (2020). BOIN12: Bayesian Optimal Interval Phase I/II Trial Design for Utility-Based Dose Finding in Immunotherapy and Targeted Therapies. JCO Precis Oncol, 4, PO.20.00257. https://doi.org/10.1200/PO.20.00257



