In the dynamic world of clinical research, selecting the right Contract Research Organization (CRO) partner is more important than ever. As clinical trials grow increasingly complex, the demand for specialized expertise and strategic partnerships is reshaping how sponsors choose their CROs. To understand what matters most in this decision-making process, we recently conducted a poll to identify the key factors influencing CRO selection.
Poll Overview: Key Drivers in CRO Selection
Our poll sought to uncover the most critical factors when selecting a CRO partner, offering industry professionals four options:
- Therapeutic expertise
- Specialized services
- Cost-effectiveness
- Strategic partnerships
These factors are all important, but understanding the hierarchy of these priorities can help provide valuable insights into current industry trends.
Key Insights from the Poll
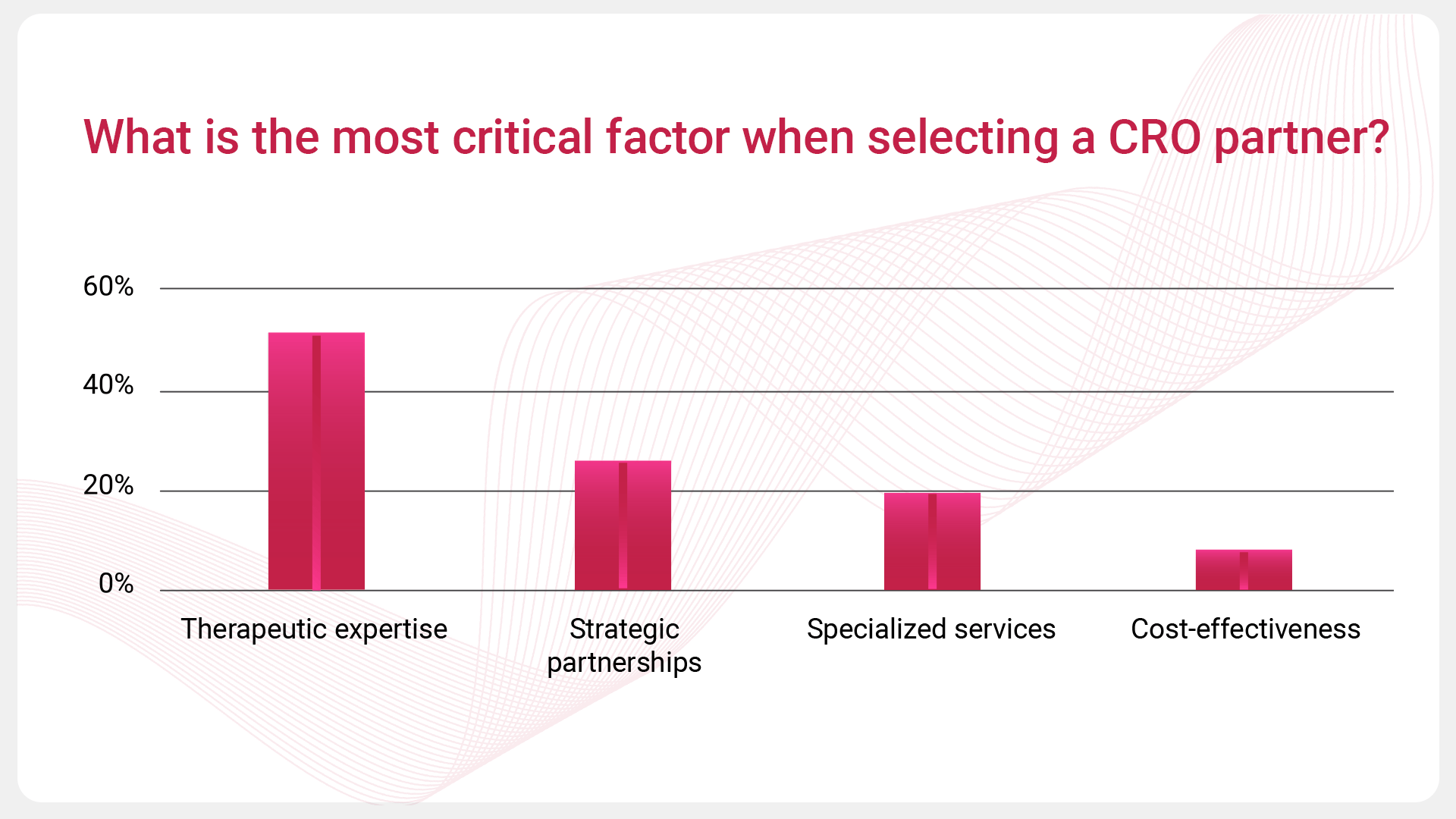
Therapeutic Expertise Takes Center Stage
Therapeutic expertise emerged as the leading priority, underscoring the value of CROs with deep knowledge in specific therapeutic areas. As clinical trials become more intricate, sponsors increasingly rely on CROs that offer specialized insights and a strong track record in their field of study.
The Importance of Strategic Partnerships
Strategic partnerships also stood out as a significant factor, coming in second place to reflect the industry’s shift towards more collaborative and long-term relationships. Sponsors are looking for CROs that align with their strategic goals, offering flexibility and the ability to adapt to the unique challenges of their trials. This highlights the growing trend of moving away from transactional interactions towards more integrated, partnership-driven approaches.
Specialized Services: A Key Differentiator
Specialized services are a critical differentiator and as such came in a close third place. In addition to therapeutic expertise, the ability of CROs to offer specialized services—enhanced by advanced technologies like data analytics and innovative trial designs—plays a crucial role in selecting the right partner. This focus on specialized capabilities reflects the industry’s demand for CROs that can meet the specific needs of complex and rapidly evolving clinical trials.
Cost-Effectiveness: A Secondary Consideration
While cost-effectiveness remains a consideration, it appears to be less critical compared to factors like expertise and strategic value. This suggests that sponsors are prioritizing the ability of CROs to deliver specialized knowledge and strategic partnerships over cost alone, recognizing that the right expertise can lead to more efficient and successful trial outcomes.
The Evolving CRO Landscape
The CRO industry has undergone significant transformation in recent years, evolving from basic service providers to strategic partners integral to the success of clinical research. By taking on complex tasks such as trial management, logistics, and data analysis, CROs have expanded the capabilities of their sponsor partners and accelerated drug development timelines.
The rise of big data analytics, precision therapies, and advanced trial technologies has further catalyzed this shift, leading sponsors to increasingly value CROs that offer specialized expertise and strategic guidance. The traditional one-size-fits-all model is being replaced by a need to align project goals with the specific strengths of a CRO, whether through specialized therapeutic knowledge, innovative trial designs, or advanced data capabilities.
Specialist CROs: Addressing Unique Challenges
Specialist CROs bring unique value through their deep therapeutic area expertise and specialized services, which are essential for navigating the diverse challenges of different research areas. In fields such as rare diseases or medical device trials, for example, specialized CROs are better equipped to handle the unique regulatory, recruitment, and technical complexities involved.
Moreover, the integration of advanced technology and data analytics has become a critical component of modern clinical trials. Specialist CROs, particularly those with strong capabilities in data science and biometrics, can offer substantial advantages by providing the expertise needed to leverage large datasets and extract valuable insights, which are increasingly crucial in today’s research environment.
Strategic Partnerships: The New Standard
The demand for more efficient and complex studies, combined with advances in personalized medicine and data-driven decision-making, is driving the shift towards closer, more strategic partnerships between sponsors and CROs. This evolving model emphasizes the need for specialized services that align closely with the sponsor’s objectives, ensuring that the right expertise is applied throughout the clinical trial process, from design to execution.
Conclusion
Selecting the right CRO partner is a critical decision that significantly impacts the success of clinical trials. Our poll results highlight the industry’s focus on therapeutic expertise, strategic partnerships, and specialized services as key factors in CRO selection. As the CRO landscape continues to evolve, these insights will guide us in refining our approach to better meet the needs of our clients.
Looking to elevate your clinical trials? If you’re in search of a CRO that excels in specialized services, strategic collaboration, and deep therapeutic knowledge, let’s explore how we can support your next trial with tailored solutions. Together, we can navigate the complexities of clinical research and achieve successful outcomes.



