Introduction
The biotech industry has become a driving force in clinical research, accelerating advances in areas such as precision medicine, oncology, and rare disease therapies. However, unlike larger pharmaceutical companies, many biotechs are challenged by limited resources and capabilities to manage complex trials, leading to a growing reliance on Clinical Research Organizations (CROs) for expertise, infrastructure, and strategic guidance.
This blog examines how biotech R&D growth, fueled by advanced therapies and data expansion, is reshaping clinical trials and market dynamics, with biotechs increasingly leveraging CROs partnerships to address challenges and drive innovation.
Biotech Growth in R&D and Market Dynamics
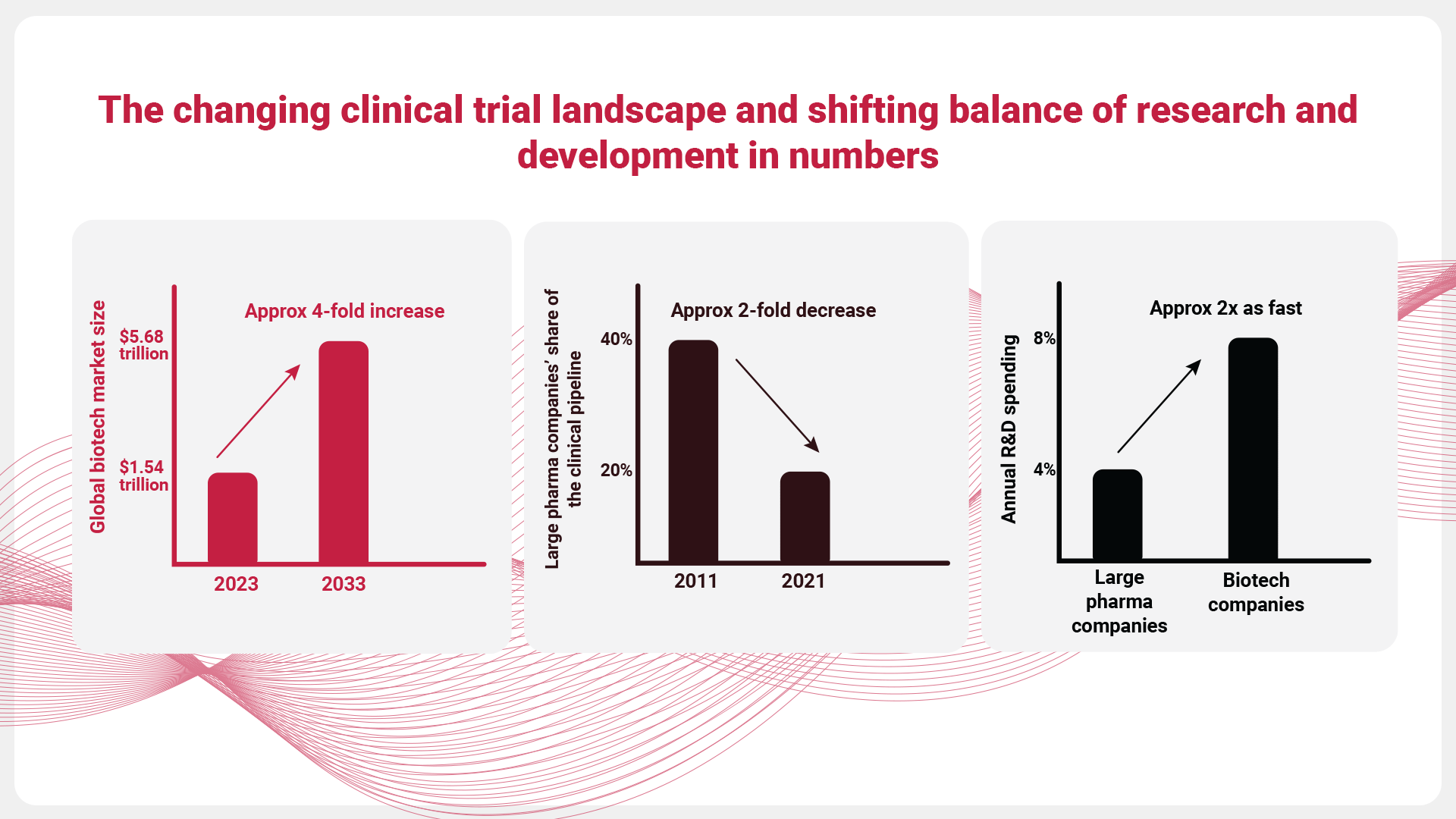
Advanced therapies, combined with global pressures and a surge in data sources, are transforming the clinical trial landscape and reshaping the balance in research and development (R&D).
From 2011 to 2021, large pharmaceutical companies saw their share of the clinical pipeline fall from approximately 40% to 20%. This trend is likely to persist, with biotech R&D spending forecasted to grow at double the pace of large pharma—8% annually compared to 4%. [1]
By 2033, the global biotech market is projected to reach USD 5.68 trillion, with a compound annual growth rate (CAGR) of 13.95% from 2024 to 2033, fueled by developments in nanobiotechnology, an increasing demand for biosimilars, and expanding applications in precision medicine. [2]
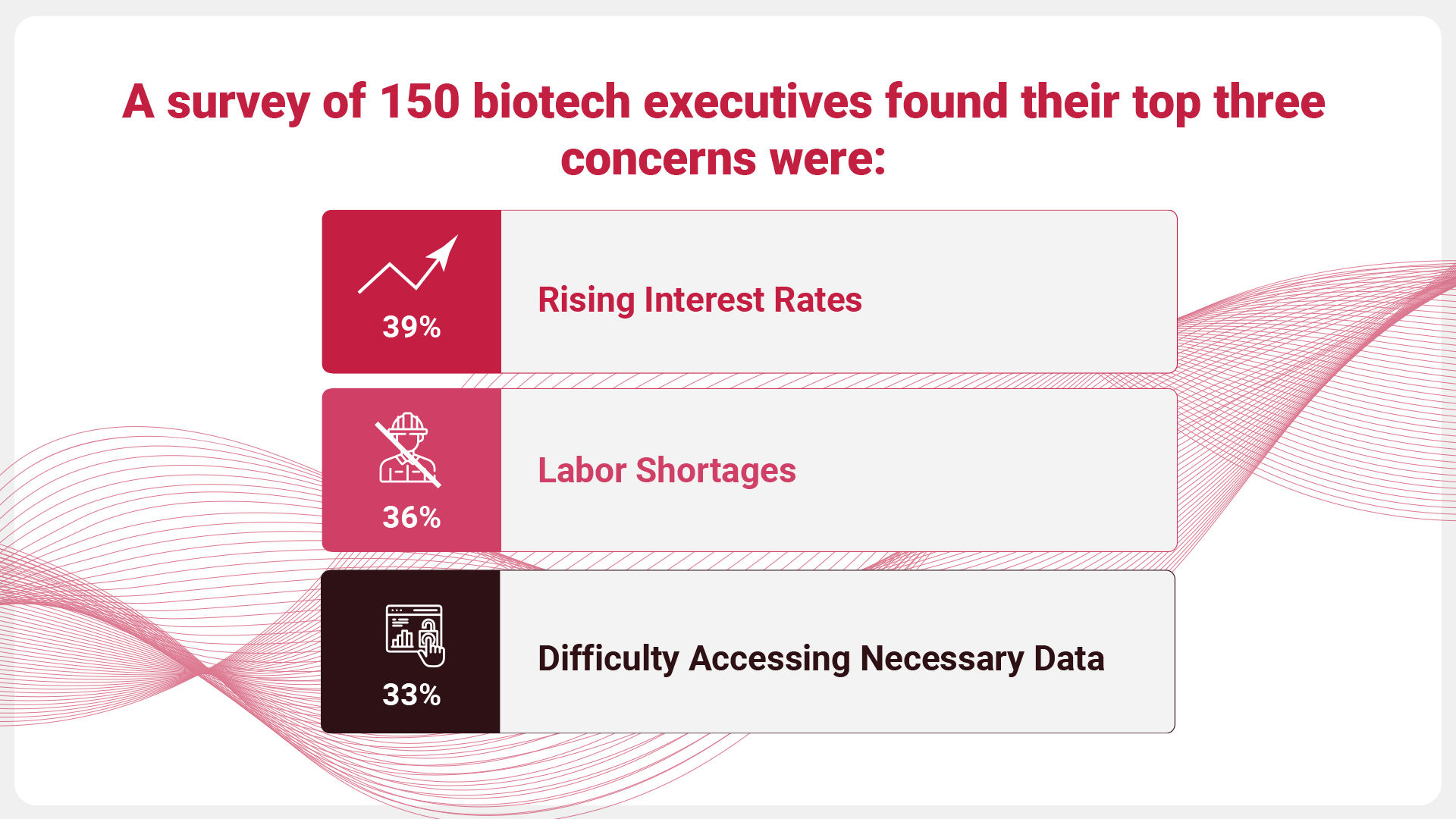
In today’s increasingly complex clinical trial landscape, biotechs are navigating global market challenges and data-related concerns. A survey of 150 biotech executives highlighted their top issues as rising interest rates (39%), labor shortages (36%), and limited access to essential data (33%). While some of these challenges may be beyond biotechs’ control, they are interconnected. For instance, improving data access and enhancing data storage and analysis systems can help reduce costs and accelerate development, making biotechs more resilient to global pressures like rising interest rates.
Addressing Potential Pitfalls in Biotech Development Through Strategic CRO Partnerships
A common solution biotechs employ to overcome challenges is to partner with a CRO; however, despite the potential benefits, small biopharma and biotech companies report lower satisfaction levels with CROs compared to their larger pharmaceutical counterparts. Qualitative interviews conducted with biotech firms indicate a perception that CROs often fall short in providing the strategic guidance and seamless integration of technology solutions that these companies require. [1]
However, when selected thoughtfully, a CRO can serve as a vital development partner for a biotech. It is essential to prioritize the areas that offer the greatest value in outsourcing and to foster genuine strategic partnerships.
Download our white paper to equip your biotech company with the knowledge needed to make confident, strategic CRO partnerships. Discover key insights, avoid common pitfalls, and learn how the right vendor can drive both efficiency and innovation in clinical development.
References
[1] McKinsey & Company. CROs and biotech companies: Fine-tuning the partnership. Retrieved from https://www.mckinsey.com/industries/life-sciences/our-insights/cros-and-biotech-companies-fine-tuning-the-partnership
[2] Nova One Advisor. Biotechnology market size, trends, and growth analysis. Retrieved from https://www.novaoneadvisor.com/report/biotechnology-market