Introduction
Risk-Based Quality Management (RBQM) has emerged as a powerful tool for optimizing clinical trial operations. While its benefits—such as near real-time data access, improved resource allocation, and enhanced patient safety—are well-known, its adoption across the industry has been slower than anticipated.
Data from the Association of Clinical Research Organizations (ACRO) highlights that the implementation of RBQM remains less widespread than expected, despite advances in clinical trial technologies. [1] This blog will explore the challenges that contribute to this slower adoption and the obstacles organizations face in successfully implementing RBQM.
Initial Setup and Integration Costs
One of the primary barriers to adopting RBQM is the significant cost and complexity of integrating it with existing Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC) platforms. Clinical trials involve various stakeholders, datasets, and systems, and introducing RBQM can be disruptive without careful planning.
Operational Hurdles
Another key challenge lies in operationalizing RBQM. Implementing this approach requires significant changes in how clinical trials are managed, including new workflows, quality management systems, and the introduction of key risk indicators (KRIs). These changes necessitate comprehensive staff training, which can be particularly difficult for organizations accustomed to traditional, established methods.
Additionally, selecting the right KRIs is a complex process. Clinical trials can involve hundreds of potential KRIs, from protocol deviations to data queries. Organizations must identify the most critical metrics to focus on, which are crucial for effective RBQM.
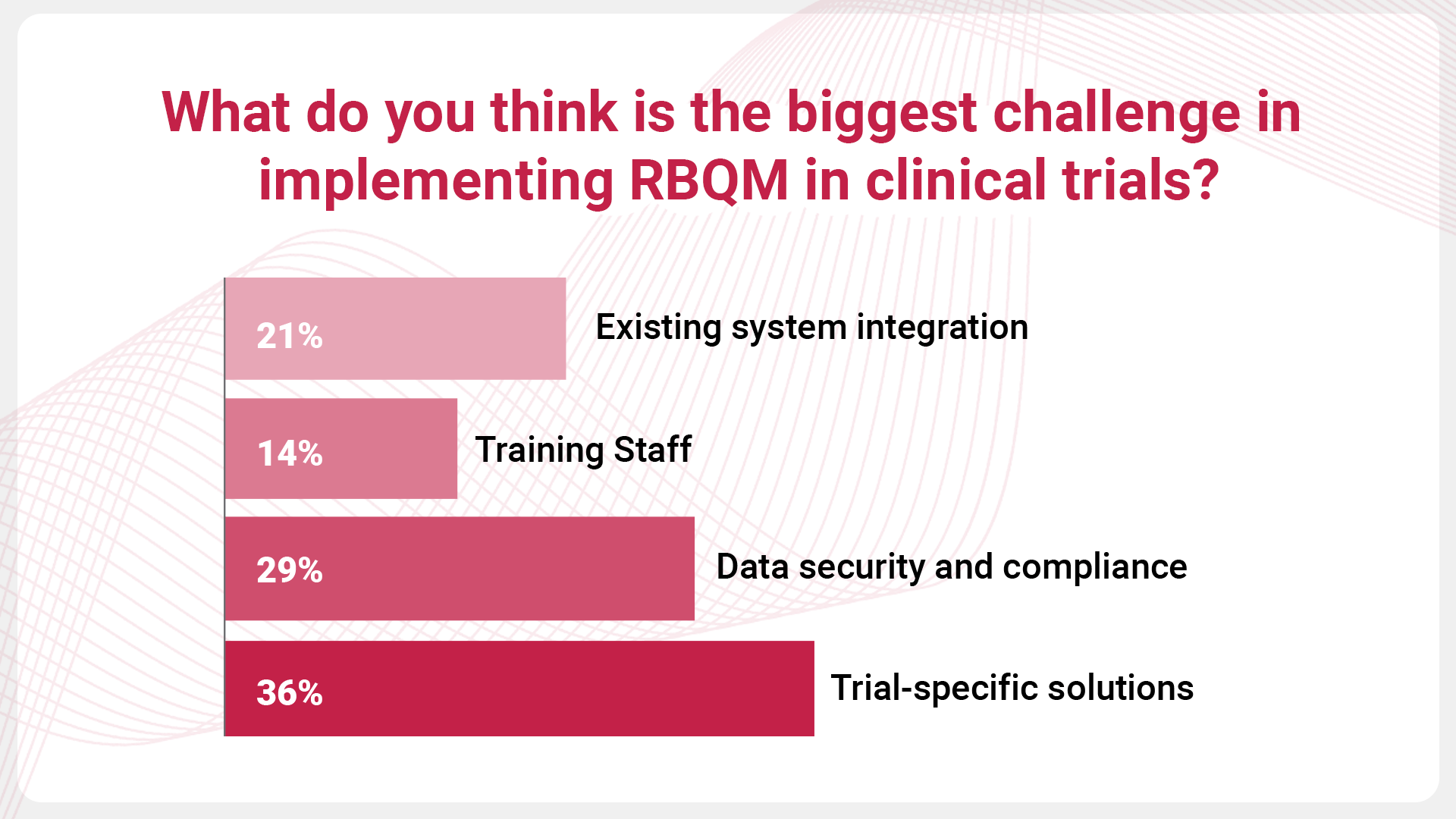
A recent survey conducted by Phastar provides further insights into the challenges faced by organizations when implementing RBQM. When asked, “What do you think is the biggest challenge in implementing RBQM in clinical trials?”, 36% of respondents highlighted trial-specific solutions as the most pressing issue, underscoring the need for tailored approaches to accommodate the unique requirements of each trial.
Many off-the-shelf RBQM solutions are not tailored to the unique needs of every trial, requiring costly customizations or the development of bespoke systems. This raises both the financial and resource demands for organizations.
Therefore, it is important to choose a partner who offers tailored, integrated RBQM solutions that fit your specific needs. At Phastar, we focus on delivering high-quality, bespoke RBQM solutions that provide long-term value.
Regulatory Compliance and Data Security
Ensuring RBQM aligns with regulatory requirements, such as GDPR in Europe and HIPAA in the U.S., is another significant hurdle. Managing data security and compliance becomes more challenging as the volume of trial data increases. Any breach or mishandling of sensitive data could compromise the trial and expose the organization to regulatory and legal risks.
Implementing RBQM requires a careful balance between innovation and strict adherence to regulatory frameworks, making close collaboration between data management, IT, and regulatory teams essential.
Stakeholder Buy-In
Successful RBQM adoption also hinges on securing buy-in from all stakeholders, including trial sponsors, CROs, and regulatory bodies. Without a shared understanding of the value and goals of RBQM, the implementation process can stall or fail to deliver its intended benefits.
Conclusion
Although RBQM offers substantial advantages in improving clinical trial oversight, its adoption comes with several challenges. High setup costs, integration complexities, operational adjustments, and regulatory compliance are significant obstacles that organizations must navigate. However, with a clear strategy and the right RBQM solution provider, these hurdles can be overcome, allowing drug developers to optimize trial outcomes and enhance patient safety.
Integrated into our Clinical Intelligence Suite, RBQM gives you near real-time data, optimizes resource use, and enhances patient safety. Additionally, our RBQM solutions are bespoke to your specific trial needs. Transform your clinical trial oversight with RBQM and unlock actionable insights.
References
[1] Dirks, A., Florez, M., Torche, F., Young, S., Slizgi, B., & Getz, K. (2024). Comprehensive assessment of risk-based quality management adoption in clinical trials. Therapeutic Innovation & Regulatory Science, 58, 520–527. https://doi.org/10.1007/s43441-024-00618-5