Introduction
The Project Optimus initiative is a key part of the U.S. Food and Drug Administration (FDA’s) Oncology Center of Excellence (OCE) and represents a shift in the agency’s approach to cancer treatment dosing. [1]
The three main goals of Project Optimus are:
- Communicating expectations for dose-finding and dose optimization
- Providing opportunities for discussions between drug developers and the FDA early in the development program of their treatment
- Developing strategies for dose finding and dose optimization using both clinical and non-clinical means, through evaluation of larger dose ranges in trials.
These goals are to ensure that more patients receive the appropriate dose that can provide the best efficacy without undue toxicity.
This blog summarizes how the FDA’s Project Optimus aims to transform oncology drug dosing. It provides a clear overview of this initiative, including how Project Optimus is catalyzing the shift from traditional dosing strategies, and explores how Project Optimus can impact clinical trial design.
Understanding Project Optimus
The landscape of oncology drug development is evolving rapidly, and with it comes the need for more precise and effective dosing strategies. Traditionally, oncology drugs have been dosed at the maximum tolerated dose (MTD), which is the highest dose that does not cause unacceptable side effects.
However, this approach assumes a linear dose-response relationship which has been repeatedly shown to be untrue, especially in oncology. [2]
To address these challenges, the FDA launched Project Optimus, an initiative aimed at transforming how oncology drugs are dosed, with a focus on optimizing efficacy and minimizing side effects. [3]
By encouraging more sophisticated dosing studies and analyses, Project Optimus aims to optimize treatment outcomes for patients and streamline the drug development process. To enhance cancer treatment, the goals of Project Optimus are to promote early and effective communication with the FDA, strategize personalized dosing approaches, and discuss optimal strategies with stakeholders at the beginning of the drug development process.
How is Project Optimus Impacting Clinical Trial Design?
Project Optimus aims to refine oncology drug dosing by emphasizing the importance of understanding the dose-response relationship more comprehensively.
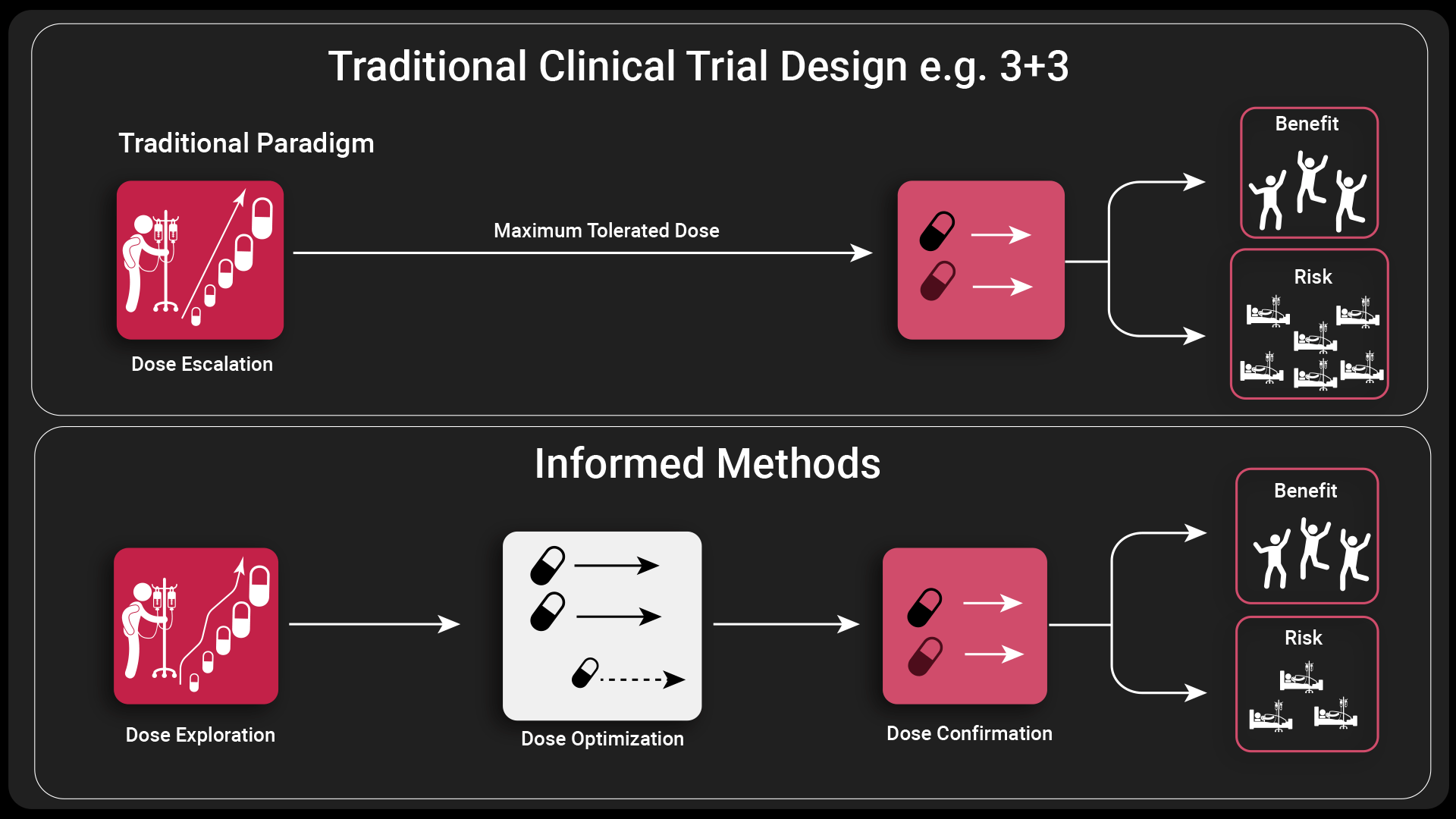
Traditional drug trials are based on the paradigm of achieving the MTD to ensure optimal efficacy. However, since a linear dose-response relationship is not a realistic paradigm and even sometimes assuming a monotonic relationship can be incorrect, often this results in patients receiving an unnecessarily toxic dose without any increased benefit in efficacy. Instead, Project Optimus looks to investigate efficacy earlier within the treatment of the development program, during dose escalation and a broader range of doses being considered into later phase trials to ensure that the most appropriate dosage is taken through to approval.
Therefore, some traditional clinical trial designs, such as the 3 +3 designs in clinical trials that determine MTD [4] will no longer provide enough information once Project Optimus is implemented. In our recent webinar our expert, Giles Partington, explored some innovative clinical trial designs that will provide sufficient information.
Exploring model-assisted and model-based design options can enhance the rigor of early-phase trials. Model-assisted approaches employ statistical models to establish predefined rules for dose escalation and de-escalation to reach optimal levels, while model-based designs typically map the entire dose-toxicity curve to inform future dose recommendations and infer the best level/range of dose to use.
Alternatively, adaptive trial designs that allow for continuous dose adjustments based on accumulating data, rather than sticking rigidly to predefined dose levels, could be used. This can result in more flexible and informative trials, accelerating the development of optimal dosing strategies and improving the overall efficiency and effectiveness of clinical research in oncology.
Summary
Project Optimus represents the FDA’s innovative approach to oncology drug dosing. Shifting away from the traditional MTD model, it focuses on personalized dosing to improve the therapeutic index and better accommodate individual patient needs.
The initiative will require advanced clinical trial designs to be considered, such as model-assisted and adaptive approaches, which allow for more precise and flexible dosing.
Thorough understanding of these factors and partnering with expert statisticians early in the clinical trial design process will ensure optimal clinical trial design, execution, accurate data analysis, and the best outcomes for your research and patients.
Look out for our next blog in the series that will explore the multiple benefits that can be gained by ensuring your clinical trial will adhere to the Project Optimus guidelines.
References
[1] Project Optimus Reforming the Dose Optimization and Dose Selection Paradigm in Oncology. U.S. Food and Drug Administration. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus
[2] Martin, J. H., & Dimmitt, S. (2019). The rationale of dose–response curves in selecting cancer drug dosing. British Journal of Clinical Pharmacology, 85(10), 2198–2204. https://doi.org/10.1111/bcp.13979
[3] Murphy, R., Halford, S., & Symeonides, S. N. (2023). Project Optimus, an FDA Initiative: Considerations for Cancer Drug Development Internationally, from an Academic Perspective. Frontiers in Oncology, 13, 1144056. https://doi.org/10.3389/fonc.2023.1144056
[4] Li, A., & Bergan, R. C. (2020). Clinical Trial Design: Past, Present, and Future in the Context of Big Data and Precision Medicine. Cancer, Wiley Online Library. https://doi.org/10.1002/cncr.33205
About Phastar
As a leading global biometrics contract research organization, we are committed to partnering with stakeholders to navigate clinical design complexities and ensure compliance with Project Optimus. Engaging with statistical experts early on and throughout the process is essential to ensure the best possible outcomes for your clinical trials.
Interested in learning more?



