Introduction
Randomized Controlled Trials (RCTs) remain the cornerstone of evidence generation in clinical research, providing rigorous and unbiased assessments of treatment efficacy and safety. The frequentist framework, which underpins most traditional trial designs, relies on predefined sample sizes and statistical significance thresholds. While effective for common conditions with large patient populations, these methods often face significant limitations in the context of rare diseases or small populations, where enrolling large cohorts is impractical or impossible. [1] Bayesian trial designs can help in such cases.
The Challenge
Rare diseases, whilst affecting a notable margin of 3.5-5.9% of the global population [1], affect relatively few individuals worldwide, usually defined as less than 5 in 10,000 people, making recruitment of the large sample sizes required for adequate statistical power challenging. This limitation can lead to prolonged recruitment periods, increased costs, and sometimes inconclusive trial results. [2] Consequently, alternative approaches and analytical frameworks capable of extracting maximal information from limited data are essential. [3]
Bayesian Trial Designs
Bayesian trial designs offer a flexible and powerful alternative to traditional frequentist approaches. Central to the Bayesian paradigm is the incorporation of prior knowledge, whether from historical data such as external data sources and previous trials, or from expert opinion, and updating this with observed trial data to generate posterior distributions that reflect an updated belief about treatment effects. This allows for adaptive trial designs where ongoing data collection informs decision-making, often reducing required sample sizes and accelerating evidence generation. [2]
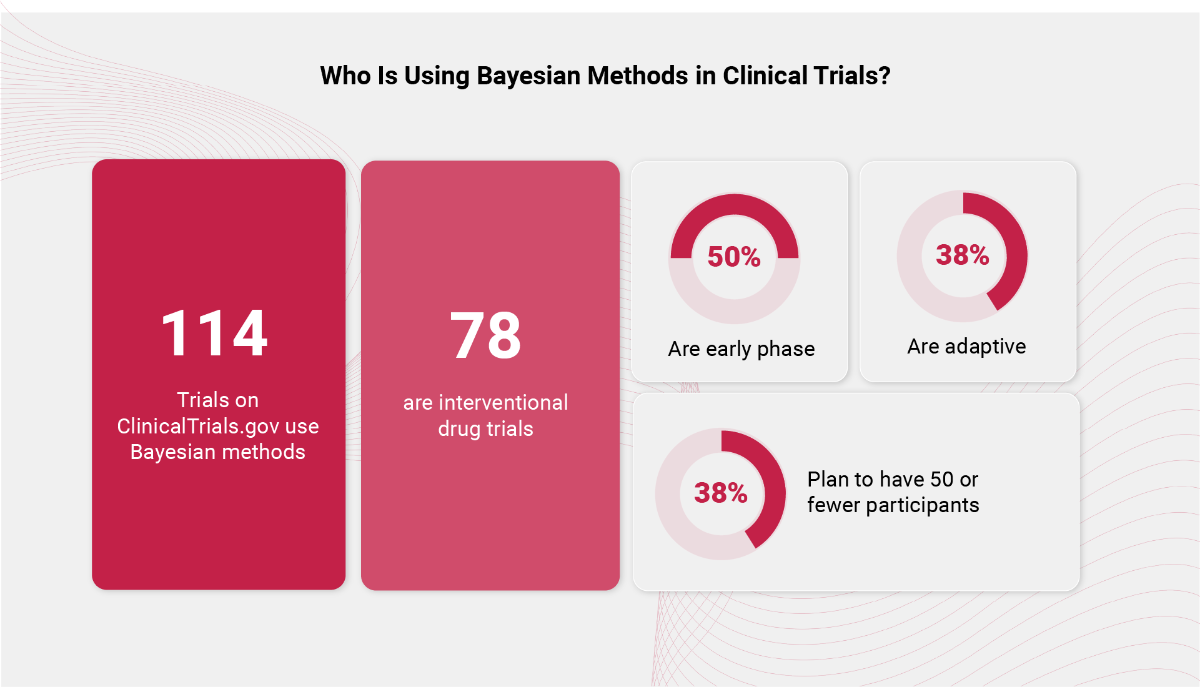
Bayesian methods are gaining traction in clinical research, particularly where data are limited and timely decision-making is essential, such as early phase trials, adaptive trials, and rare disease/small population trials. An ongoing review of ClinicalTrials.gov reveals 114 trials have or are using Bayesian approaches, with 78 of these focused on interventional drug studies. Notably, half of these trials are in early phases of development, where flexibility and efficiency are key. Additionally, 38% employ adaptive designs, and the same proportion plan to enroll 50 or fewer participants, highlighting the growing use of Bayesian frameworks in small, agile studies where traditional methods may fall short.
Expert Priors
In many rare-disease trials, historical data are limited or unavailable. Structured expert elicitation transforms clinical expertise into formal prior probability distributions that incorporate uncertainty. Techniques like the SHELF (Sheffield Elicitation Framework) protocol have been successfully applied to obtain robust priors from domain experts in a repeatable and clearly recorded manner. [4] This integration of expert knowledge helps ensure Bayesian analyses are scientifically rigorous and relevant.
Regulatory Considerations for Bayesian Trial Designs
The FDA acknowledges Bayesian designs as acceptable when thoroughly justified with transparent methodology and supported by simulation studies demonstrating robust operating characteristics. Guidance documents emphasize the need for comprehensive statistical plans and evidence dossiers to facilitate regulatory review and approval.[5]
Conclusion
As clinical trials increasingly focus on rare diseases and small populations, Bayesian methods provide a scientifically sound and practical approach to evidence generation. By combining prior knowledge and adaptive strategies, these methods address key limitations of traditional frequentist designs, supporting more efficient and informative trials.
To explore further, listen to our recent expert-led webinar covering Bayesian methodologies, challenges, and real-world applications in clinical research.
References
1.Partington, G., Cro, S., Mason, A., Phillips, R., & Cornelius, V. (2022). Design and analysis features used in small population and rare disease trials: A targeted review. Journal of Clinical Epidemiology, 144, 93–101. https://doi.org/10.1016/j.jclinepi.2021.12.009
2.Nguengang Wakap, S., Lambert, D. M., Olry, A., Rodwell, C., Gueydan, C., Lanneau, V., Murphy, D., Le Cam, Y., & Rath, A. (2020). Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. European Journal of Human Genetics, 28(2), 165–173. https://doi.org/10.1038/s41431-019-0508-0
3.Parmar, M., Sydes, M., & Morris, T. (2016). How do you design randomised trials for smaller populations? A framework. BMC Medicine, 14, 183. https://doi.org/10.1186/s12916-016-0734-z
4.Gosling, J. P. (2018). SHELF: The Sheffield Elicitation Framework. In L. C. Dias, A. Morton, & J. Quigley (Eds.), Elicitation (International Series in Operations Research & Management Science, Vol. 261, pp. 61–93). Springer. https://doi.org/10.1007/978-3-319-65052-4_4
5.FDA. (2010). Guidance for Industry: Adaptive Design Clinical Trials for Drugs and Biologics. U.S. Food and Drug Administration. https://www.fda.gov/media/78495/download